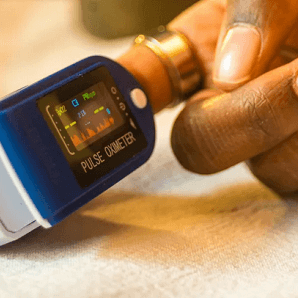
The FDA, a government agency that regulates medical devices, has asked companies that make pulse oximeters to include more people with darker skin in their testing. Pulse oximeters are small devices that measure the amount of oxygen in a person’s blood by attaching to their finger.
Recent studies have shown that pulse oximeters may not work as well for people with darker skin compared to those with lighter skin. This means that Black, Hispanic, and Asian patients might not receive the right amount of oxygen when they are sick, which could lead to unfair differences in their medical care.
In one study from 2022, Black patients were more likely than White patients to have low blood oxygen levels that were not detected by pulse oximeters. Many doctors, like Dr. Dionne Ibekie, didn’t know about this problem until recently.
To fix this issue, the FDA wants pulse oximeter companies to test their devices on people with a wide range of skin colors and to make sure they work well for everyone over time. By doing this, the FDA hopes to make sure that all patients, no matter their skin color, receive fair and equal medical care.
See “FDA panel recommends more diversity in pulse oximeter trials” by Jacqueline Howard on the CNN website (February 2, 2024)